Abstract
Background
This randomized double-blinded trial compared the effect of intravenous and perineural dexamethasone (8 mg) on the duration of motor block for ultrasound (US)-guided axillary brachial plexus block (AXB).
Methods
Patients undergoing upper limb surgery with US-guided AXB were randomly allocated to receive preservative-free dexamethasone (8 mg) via intravenous (n = 75) or perineural (n = 75) administration. The local anesthetic agent, 1% lidocaine −0.25% bupivacaine (30 mL) with epinephrine 5 µg·mL−1, was identical in all subjects. Operators and patients were blinded to the nature of the intravenous and perineural injectate. A blinded observer assessed the block success rate (i.e., a minimal sensorimotor composite score of 14 out of 16 points at 30 min), block onset time, as well as the presence of surgical anesthesia. Postoperatively, the blinded observer contacted all patients with successful blocks to record the duration of motor block (primary outcome), sensory block, and postoperative analgesia.
Results
No intergroup differences were observed in terms of success rate, surgical anesthesia, and block onset time. Compared to intravenous administration, perineural dexamethasone provided longer mean (SD) durations for motor block [17.5 (4.6) hr vs 12.8 (4.5) hr; mean difference, 4.6 hr; 95% confidence interval [CI], −6.21 to −3.08; P < 0.001], sensory block [17.7 (5.1) hr vs 13.7 (5.0) hr; mean difference, 4.0 hr; 95% CI, −5.77 to −2.27; P < 0.001], and postoperative analgesia [21.1 (4.6) hr vs 17.1 (4.6) hr; mean difference, 4.0 hr; 95% CI, −5.70 to −2.30; P < 0.001].
Conclusion
Compared to intravenous dosing, perineural dexamethasone (8 mg) results in longer durations of sensorimotor block and postoperative analgesia for ultrasound-guided axillary block. This trial was registered at www.clinicaltrials.gov number, NCT02629835.
Résumé
Contexte
Cette étude randomisée à double insu portant sur le bloc axillaire du plexus brachial par échoguidage a comparé l’effet de la dexaméthasone (8 mg) administrée par voie intraveineuse ou périneurale sur la durée du bloc moteur.
Méthode
Des patients subissant une chirurgie des membres supérieurs sous un bloc axillaire du plexus brachial réalisé par échoguidage ont été randomisés à recevoir une dose de dexaméthasone (8 mg) sans agent de conservation par administration intraveineuse (n = 75) ou périneurale (n = 75). L’anesthésique local était identique pour tous les patients de l’étude, lidocaïne 1% et bupivacaïne −0,25% (30 mL) avec 5 µg·mL−1 d’épinéphrine. Ni les opérateurs, ni les patients n’étaient informés de la nature de l’injectat, soit intraveineux ou périneural. Un observateur en aveugle a évalué divers facteurs : le taux de réussite du bloc (soit un résultat composé sensitivo-moteur minimal de 14 sur 16 points à 30 min), le moment d’amorce du bloc, et la présence d’une anesthésie chirurgicale. En période postopératoire, l’observateur en aveugle a contacté tous les patients dont le bloc était réussi afin d’enregistrer la durée du bloc moteur (critère d’évaluation principal), le bloc sensitif et l’analgésie postopératoire.
Résultats
Aucune différence n’a été observée entre les groupes en matière de taux de réussite, d’anesthésie chirurgicale, ou d’amorce du bloc. Par rapport à l’administration intraveineuse, la dexaméthasone administrée par voie périneurale a procuré une durée moyenne (ÉT) prolongée du bloc moteur [17,5 (4,6) h vs 12,8 (4,5) h; différence moyenne, 4,6 h; intervalle de confiance [IC] 95%, −6,21 à −3,08; P < 0,001], du bloc sensitif [17,7 (5,1) h vs 13,7 (5,0) h; différence moyenne, 4,0 h; IC 95%, −5,77 à −2,27; P < 0,001] et d’analgésie postopératoire [21,1 (4,6) h vs 17,1 (4,6) h; différence moyenne, 4,0 h; IC 95%, −5,70 à −2,30; P < 0,001].
Conclusion
Par rapport à la posologie intraveineuse, la dexaméthasone périneurale (8 mg) entraîne une durée prolongée du bloc sensitivo-moteur et de l’analgésie postopératoire lors d’un bloc axillaire échoguidé. Cette étude a été enregistrée au www.clinicaltrials.gov, numéro NCT02629835.
Similar content being viewed by others
Dexamethasone is a common adjuvant for interscalene,1-5 supraclavicular,6-16 and axillary17-19 brachial plexus blocks. However, the optimal method of administration remains unknown. In a recent multicentre trial comparing intravenous (IV) and perineural (PN) administration of dexamethasone for ultrasound (US)-guided infraclavicular block (n = 150), we showed that the PN modality provided longer sensorimotor block and analgesia.20 Nevertheless, other trials have failed to detect significant differences between IV and PN dexamethasone.5,16,21,22 We speculated that these contradictory findings in the literature may stem from differences in the doses of dexamethasone and local anesthetic (LA) used as well as insufficient statistical power due to small sample sizes. In addition, different nerve blocks may respond differently to IV and PN dexamethasone.20
Accordingly, we designed the current trial as a follow-up to our previous study which demonstrated prolonged sensorimotor blockade with PN dexamethasone for infraclavicular block.20 In order to validate our previous findings, we sought again to compare IV and PN dexamethasone, by using a different (i.e., axillary) approach to the brachial plexus. We selected motor block duration as the primary outcome because the latter is arguably more objective than sensory blockade since analgesic and sensory duration can be influenced by concomitant pain medications and surgical trauma to small cutaneous nerves, respectively. We hypothesized that, compared to IV dosing, PN administration of dexamethasone would result in a longer motor block for patients undergoing US-guided axillary brachial plexus block (AXB).
Methods
After securing institutional ethics committee approval [McGill University Health Centre, Montreal, QC, Canada (29/1/2016) and Maharaj Nakorn Chiang Mai Hospital, Chiang Mai, Thailand (27/1/2016)] and obtaining written informed consent, we recruited 150 patients scheduled for surgery of the forearm, wrist, or hand. Inclusion criteria were patients aged 18-80 yr, American Society of Anesthesiologists physical status I-III, and body mass index 18-35 kg·m−2. Exclusion criteria included sepsis, coagulopathy, allergy to LA, hepatic or renal failure, preexisting upper limb neuropathy, and prior surgery in the axilla. After arrival in the anesthesia induction room, IV access was secured in the non-surgical upper extremity. Intravenous premedication (midazolam 0.015-0.03 mg·kg−1 and fentanyl 0.6 μg·kg−1) was administered to all subjects. Supplemental oxygen (nasal cannulae at 4 L·min−1) and pulse oximetry were applied throughout the procedure. All AXBs were performed by residents, fellows, or staff anesthesiologists. Operators were considered experts if they possessed a minimal experience of 60 US-guided AXBs.23
The AXB was performed according to a previously described technique.24-26 All patients received 1.0% lidocaine-0.25% bupivacaine (obtained by mixing equal parts of 2% lidocaine and 0.5% bupivacaine) with epinephrine 5 μg·mL−1. A SonoSite M-Turbo™ US machine (SonoSite Inc, Bothell, WA, USA) and a 6-13 MHz linear US probe were used in all subjects. We placed patients in a supine position with the shoulder abducted and the elbow flexed. The US probe was applied in the axilla in a sterile fashion in order to obtain a short-axis view of the musculocutaneous nerve and axillary artery. A skin wheal was raised with 3 mL of 1% lidocaine. Subsequently, using an in-plane US technique and a lateral-to-medial direction, a 22G 5-cm block needle (StimuQuik® ECHO; Arrow® International Inc, Reading, PA, USA) was advanced towards the musculocutaneous nerve, and 6 mL of the LA mixture were deposited around the latter. The needle was then advanced until its tip was positioned just dorsal to the axillary artery. Twenty four mL of LA were incrementally injected in this location. A “silhouette sign” – defined as blurring of the arterial wall due to the contiguity of anechoic blood and anechoic LA – 24-26 was sought to ensure proximity between artery and needle tip.
Patients were allocated to receive IV or PN dexamethasone (8 mg) using a computer-generated randomization sequence and sealed envelopes. All envelopes were kept secured by the study coordinators in the two centres and were opened in the anesthesia induction room only after the patient provided written consent for study participation. Randomization was carried out in blocks of 50 subjects at each of the two centres to ensure an equal distribution between groups.
In the PN group, 0.8 mL of preservative-free dexamethasone (10 mg·mL−1) was administered with the LA and 0.8 mL of normal saline was injected intravenously. Conversely, in the IV group, 0.8 mL of normal saline was administered with the LA, and 0.8 mL of preservative-free dexamethasone (10 mg·mL−1) was injected intravenously. An investigator not involved in clinical care prepared the study solutions in order to preserve blinding of patients and operators.
For both groups, the performance time was defined as the interval from US probe-patient contact to the end of LA injection through the block needle. The number of needle passes was also recorded, with the initial needle insertion counting as the first pass. Any subsequent advancement (preceded by a 1-cm retraction) counted as an additional pass.27 Furthermore, procedural pain scores (0 = no pain; 10 = worst imaginable pain), unintentional vascular puncture, and (any) paresthesia were also recorded. An anesthesia assistant not otherwise involved in patient care recorded the performance time, while the attending anesthesiologist supervising the block, who was blinded to group assignment, assessed the number of passes, procedural pain, vascular puncture, and the occurrence of paresthesia.
After LA injection through the block needle, an observer blinded to group allocation measured brachial plexus blockade every five minutes until 30 min. Sensory block of the musculocutaneous, median, radial, and ulnar nerves was graded according to a three-point scale using a cold test: 0 = no block; 1 = analgesia (patient can feel touch but not cold sensation); 2 = anesthesia (patient cannot feel touch).20,24-26 The musculocutaneous, median, radial, and ulnar nerves were assessed on the lateral aspect of the forearm, the volar aspect of the thumb, the lateral aspect of the dorsum of the hand, and the volar aspect of the fifth finger, respectively.20,24-26 Motor block was also graded on a three-point scale: 0 = no block; 1 = paresis; 2 = paralysis.20,24-26 The musculocutaneous, radial, median, and ulnar nerves were evaluated by elbow flexion, thumb abduction, thumb opposition, and thumb adduction, respectively.20,24-26 Overall, the maximal composite score was 16 points. We considered the AXB successful and the patient ready for surgery when a minimal composite score of 14 points was achieved – a definition and scale which have been used in previous studies.20,24-26 The onset time was defined as the time required to obtain 14 points. If the composite score was inferior to 14 points after 30 min, we considered the block to have failed and did not record an onset time for these subjects. After 30 min, all patients were transferred to the operating room for the start of surgery. The blinded observer recorded the presence of surgical anesthesia, which was defined as the ability to proceed with surgery without the need for IV narcotics, general anesthesia, rescue blocks, or LA infiltration by the surgeon.20,24-26 However, in case of anxiety (as voiced by patients or determined by the blinded treating anesthesiologists), subjects could receive an intraoperative infusion of propofol (25-80 μg·kg−1·min−1) provided response to verbal stimulus was maintained.
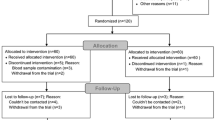
Postoperatively, patients with successful blocks (i.e., minimal composite score of 14 points at 30 min) were instructed to record the exact time they first regained movement of their fingers (i.e., duration of motor block) and sensation in their fingers (i.e., duration of sensory block) and experienced pain at the surgical site (i.e., analgesic duration).20 We did not require movement or sensation of specific digits but only in those whose tips were not covered by the cast. The blinded observer contacted study subjects at 24 hr for data collection. In the event that patients could not be reached or their AXB had receded during sleep, we did not record any data for the duration of sensorimotor block or postoperative analgesia. However, the results for technical execution and presence of a successful block were retained for analysis (Fig. 1).
CONSORT diagram of patient flow through the study. Onset times could not be recorded for patients with minimal composite scores < 14 points at 30 minutes. However, the performance time, number of needle passes, procedural pain, operator’s experience level, adverse events (vascular puncture/paresthesia), and surgical anesthesia were recorded for these subjects. IV = intravenous; PN = perineural
One week after the surgery, a blinded observer contacted patients again to inquire about complications, including persistent numbness, paresthesia, and motor deficit.
Statistical analysis
In a preliminary pilot study (undertaken to provide information for subsequent sample size calculations), we found that PN dexamethasone (8 mg) provided a mean (SD) motor block duration of 934 (282) min for US-guided AXB. Based on these values, a 150-min (2.5-hr) difference, which we considered clinically significant, would represent an effect size of 0.53 and would require 57 patients per group for an alpha error of 0.05 and a beta error of 0.20 (Student’s t test). As block duration can be calculated only for successful blocks and since we anticipated a 90% success rate with a 30-mL volume of LA,26 127 subjects were needed to account for block failure. Furthermore, as the duration of motor block cannot be accurately measured if the AXB recedes during the patient’s sleep (estimated to be approximately 15% of cases), 150 patients were enrolled to compensate for this potential dropout.
Statistical analysis was performed using SPSS® version 21 statistical software (IBM, Armonk, NY, USA). For continuous data, normality was first assessed with the Lilliefors test and then analyzed with the Student’s t test. Data not normally distributed as well as ordinal data were analyzed with the Mann-Whitney U test. For categorical data, the Chi square or Fisher’s exact test was used. The log-rank test was used to analyze the Kaplan-Meier plots for the duration of analgesia as well as sensory and motor blocks. All reported P values are two sided.
Results
The 150 subjects were recruited from January-May 2016 (Fig. 1); 100 patients were enrolled in Montreal and 50 were recruited in Chiang Mai. Patient demographic data are presented in Table 1.
Technical execution (i.e., success rate, surgical anesthesia, performance time, onset time, number of needle passes, vascular puncture, paresthesia, and procedural pain) was similar between the two groups (Table 2) (Fig. 2).
Compared to IV administration, PN dexamethasone provided longer mean (SD) durations for motor block [17.5 (4.6) hr vs 12.8 (4.5) hr; mean difference, 4.6 hr; 95% confidence interval [CI], −6.21 to −3.08; P < 0.001], sensory block [17.7 (5.1) hr vs 13.7 (5.0) hr; mean difference, 4.0 hr; 95% CI, −5.77 to −2.27; P < 0.001], and postoperative analgesia [21.1 (4.6) hr vs 17.1 (4.6) hr; mean difference, 4.0 hr; 95% CI, −5.70 to −2.30; P < 0.001] (Table 3) (Figs. 3, 4, 5).
Patient follow-up at one week revealed no motor deficit. In the IV group, one subject reported residual digital paresthesiae, but all symptoms had spontaneously resolved by the one-month follow-up.
Discussion
In this randomized trial, we compared IV and PN dexamethasone for US-guided AXB. Our current results validate our previous findings20 and again suggest that, compared to IV dosing, PN administration provides longer motor block, sensory block, and postoperative analgesia. The 4.0-4.7 hr increases in block duration/analgesia represent 23-38% increments in offset times and could offer substantial benefits for outpatient upper limb surgery. For example, this could translate into uninterrupted sleep for the patient compared with sudden awakening in the middle of the night because of pain.
Despite its widespread use,1-22 dexamethasone remains officially an off-label PN adjuvant. Although safety concerns have been voiced regarding possible toxicity, any potential corticosteroid-related neurotoxicity is likely due to preservatives (e.g., benzyl alcohol preservative, polyethylene glycol) or particulates in the preparation.5 In this study, we intentionally used a preservative-free formulation of dexamethasone, as preliminary evidence suggests that the latter may protect against LA-induced neurotoxicity.28
The optimal dose of PN dexamethasone remains unknown.29 For interscalene and infraclavicular blocks, 4-5 mg of PN dexamethasone have been shown to provide a longer duration of sensorimotor block and/or analgesia than their IV counterparts.4,20 In contrast, all studies using 8-10 mg have found similar block durations for the two modalities.5,19,21 Thus, we cannot rule out the possibility that higher dexamethasone doses could selectively favour the IV route thereby achieving parity with PN administration. Therefore, in the current trial, we erred on the side of caution and, by using 8 mg (and intentionally favouring the IV group), we sought to determine if the PN route truly outperforms IV administration.
Our AXB technique deserves special mention. We only specifically targeted the musculocutaneous nerve; as the LA was injected dorsal to the axillary artery, blockade of the median, radial, and ulnar nerves was most likely achieved through LA diffusion. Some practitioners may prefer a more targeted perineural technique whereby the four terminal nerves are painstakingly identified and anesthetized. However, in a previous randomized trial, we have compared perivascular and perineural US-guided AXB and observed that, despite similar success rates and total anesthesia-related times, the perivascular technique provided greater ease of performance (i.e., shorter performance time and fewer needle passes).24 Thus, in our practice, the perivascular method has become standard of care for performing US-guided AXB.
The lack of a control group also requires discussion. In our study, all patients received dexamethasone (IV or PN). We elected not to enroll a control group (i.e., IV and PN normal saline) because both Movafegh et al. 17 and Yaghoobi et al.18 have previously demonstrated longer AXB duration with PN dexamethasone compared to normal saline. Therefore, we echo Rosenfeld et al.’s position,5 which suggests that, in light of the cumulated evidence,1,2,5-18 a normal saline control group is no longer required, and statistical power would be better served if all subjects were allocated to receive either IV or PN dexamethasone. Furthermore, as our clinical practice now includes routine dexamethasone, we feared that its omission solely for research purposes might have breached clinical equipoise.
Our study does present some limitations. First, the durations of sensorimotor block and postoperative analgesia inherently depended on patient recall; therefore, in order to minimize subjectivity, we selected motor block as the primary outcome. Furthermore, we discarded subjects whose blocks wore off during their sleep. Second, our findings are specific to the lidocaine-bupivacaine mixture employed. Further trials are required for other LA solutions. Finally, we did not record breakthrough opioid consumption because the trial was carried out in two different countries: we reasoned that different patterns of opioid prescription (and consumption) might have constituted a confounding variable.20 Although opioids could influence the analgesic duration, they should have no impact on our primary outcome (duration of motor block).
In conclusion, compared with its IV counterpart, PN dexamethasone (8 mg) provides longer sensorimotor block and better postoperative analgesia for US-guided AXB.
References
Vieira PA, Pulai I, Tsao GC, Manikantan P, Keller B, Connelly NR. Dexamethasone with bupivacaine increases duration of analgesia in ultrasound-guided interscalene brachial plexus blockade. Eur J Anaesthesiol 2010; 27: 285-8.
Tandoc MN, Fan L, Kolesnikov S, Kruglov A, Nader ND. Adjuvant dexamethasone with bupivacaine prolongs the duration of interscalene block: a prospective, randomized trial. J Anesth 2011; 25: 704-9.
Cummings KC 3rd, Napierkowsky DE, Parra-Sanchez I, et al. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth 2011; 107: 446-53.
Kawanishi R, Yamamoto K, Tobetto Y, et al. Perineural but not systemic low-dose dexamethasone prolongs the duration of interscalene block with ropivacaine: a prospective randomized trial. Local Reg Anesth 2014; 7: 5-9.
Rosenfeld DM, Ivancic MG, Hattrup SJ, et al. Perineural versus intravenous dexamethasone as adjuncts to local anaesthetic brachial plexus block for shoulder surgery. Anaesthesia 2016; 71: 380-8.
Shrestha BR, Maharjan SK, Tabedar S. Supraclavicular brachial plexus block with and without dexamethasone - a comparative study. Kathmandu Univ Med J (KUMJ) 2003; 1: 158-60.
Yadav RK, Sah BP, Kumar P, Singh SN. Effectiveness of addition of neostigmine or dexamethasone to local anaesthetic in providing perioperative analgesia for brachial plexus block: a prospective, randomized, double blinded, controlled study. Kathmandu Univ Med J (KUMJ) 2008; 6: 302-9.
Golwala MP, Swadia VN, Dhimar AA, Sridhar NV. Pain relief by dexamethasone as an adjuvant to local anaesthetics in supraclavicular brachial plexus block. J Anaesthesiol Clin Pharmacol 2009; 25: 285-8.
Parrington SJ, O’Donnell D, Chan VW, et al. Dexamethasone added to mepivacaine prolongs the duration of analgesia after supraclavicular brachial plexus blockade. Reg Anesth Pain Med 2010; 35: 422-6.
Islam SM, Hossain MH, Maruf AA. Effect of addition of dexamethasone to local anaesthetics in supraclavicular brachial plexus block. JAFMC Bangladesh 2011; 7: 11-4.
Biradar PA, Kaimar P, Gopalakrishna K. Effect of dexamethasone added to lidocaine in supraclavicular brachial plexus block: a prospective, randomized, double-blind study. Indian J Anaesth 2013; 57: 180-4.
Dar FA, Najar MR, Jan N. Effect of addition of dexamethasone to ropivacaine in supraclavicular brachial plexus block. Indian J Pain 2013; 27: 165-9.
Persec J, Persec Z, Kopljar M, et al. Low-dose dexamethasone with levobupivacaine improves analgesia after supraclavicular brachial plexus blockade. Int Orthop 2014; 38: 101-5.
Kumar S, Palaria U, Sinha AK, Punera DC, Pandey V. Comparative evaluation of ropivacaine and ropivacaine with dexamethasone in supraclavicular brachial plexus block for postoperative analgesia. Anesth Essays Res 2014; 8: 202-8.
Ganvit KS, Akshay HM, Singhal I, Upadhyay MR. The efficacy of dexamethasone added as an adjuvant to ropivacaine (0.5%) for brachial plexus block. Int J Res Med 2014; 3: 71-4.
Abdallah FW, Johnson J, Chan V, et al. Intravenous dexamethasone and perineural dexamethasone similarly prolong the duration of analgesia after supraclavicular brachial plexus block: a randomized, triple-arm, double-blind, placebo-controlled trial. Reg Anesth Pain Med 2015; 40: 125-32.
Movafegh A, Razazian M, Hajimaohamadi F, Meysamie A. Dexamethasone added to lidocaine prolongs axillary brachial plexus blockade. Anesth Analg 2006; 102: 263-7.
Yaghoobi S, Seddighi M, Yazdi Z, Ghafouri R, Khezri MB. Comparison of postoperative analgesic effect of dexamethasone and fentanyl added to lidocaine through axillary block in forearm fracture. Pain Res Treat 2013; 2013: 761583.
Saritas A, Sabuncu C. Comparison of clinical effects of prilocaine, dexamethasone added to prilocaine and levobupivacaine on brachial plexus block. J Pak Med Assoc 2014; 64: 433-6.
Leurcharusmee P, Aliste J, Van Zundert TC, et al. A multicenter randomized comparison between intravenous and perineural dexamethasone for ultrasound-guided infraclavicular block. Reg Anesth Pain Med 2016; 41: 328-33.
Desmet M, Braems H, Reynvoet M, et al. I.V. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: a prospective, randomized, placebo-controlled study. Br J Anaesth 2013; 111: 445-52.
Rahangdale R, Kendall MC, McCarthy RJ, et al. The effects of perineural versus intravenous dexamethasone on sciatic nerve blockade outcomes: a randomized, double-blind, placebo-controlled study. Anesth Analg 2014; 118: 1113-9.
Konrad C, Schupfer G, Wietlisbach M, Gerber H. Learning manual skills in anesthesiology: is there a recommended number of cases for anesthetic procedures? Anesth Analg 1998; 86: 635-9.
Tran DQ, Pham K, Dugani S, Finlayson RJ. A prospective, randomized comparison between double-, triple-, and quadruple-injection ultrasound-guided axillary brachial plexus block. Reg Anesth Pain Med 2012; 37: 248-53.
Bernucci F, González AP, Finlayson RJ, Tran DQ. A prospective, randomized comparison between perivascular and perineural ultrasound-guided axillary brachial plexus block. Reg Anesth Pain Med 2012; 37: 473-7.
Gonzalez AP, Bernucci F, Pham K, Finlayson RJ, Tran DQ. Minimum effective volume of lidocaine for double-injection ultrasound-guided axillary block. Reg Anesth Pain Med 2013; 38: 16-20.
Casati A, Danelli G, Baciarello M, et al. A prospective, randomized comparison between ultrasound and nerve stimulation guidance for multiple injection axillary brachial plexus block. Anesthesiology 2007; 106: 992-6.
Ma R, Wang X, Lu C, et al. Dexamethasone attenuated bupivacaine-induced neuron injury in vitro through a threonine-serine protein kinase B-dependent mechanism. Neuroscience 2010; 167: 329-42.
Tighe SQ. Perineural or intravenous dexamethasone: do we still need catheters? Anaesthesia 2006; 71: 983-4.
Acknowledgements
The authors thank Mr. Derek Mitchell as well as Drs Richard Bondy, Sonia Ah-Kye, Andrew Owen, Richard Robinson, and Gina Wu for their invaluable assistance with patient recruitment.
Funding
The authors did not receive external funding for this study.
Conflicts of interests
None declared.
Author contributions
Julian Aliste, Tom C.R.V. Van Zundert, Roderick J. Finlayson, and De Q.H. Tran designed the trial and collected the pilot data. Julian Aliste, Prangmalee Leurcharusmee, Phatthanaphol Engsusophon, Aida Gordon, Giuliano Michelagnoli, Chonticha Sriparkdee, Worakamol Tiyaprasertkul, Dana Q. Tran, Roderick J. Finlayson, and De Q.H. Tran helped conduct the study as well as collect and analyze the data. Julian Aliste, Prangmalee Leurcharusmee, Roderick J. Finlayson, and De Q.H. Tran helped write the manuscript. All 11 authors reviewed the analysis of the data.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aliste, J., Leurcharusmee, P., Engsusophon, P. et al. A randomized comparison between intravenous and perineural dexamethasone for ultrasound-guided axillary block. Can J Anesth/J Can Anesth 64, 29–36 (2017). https://doi.org/10.1007/s12630-016-0741-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-016-0741-8